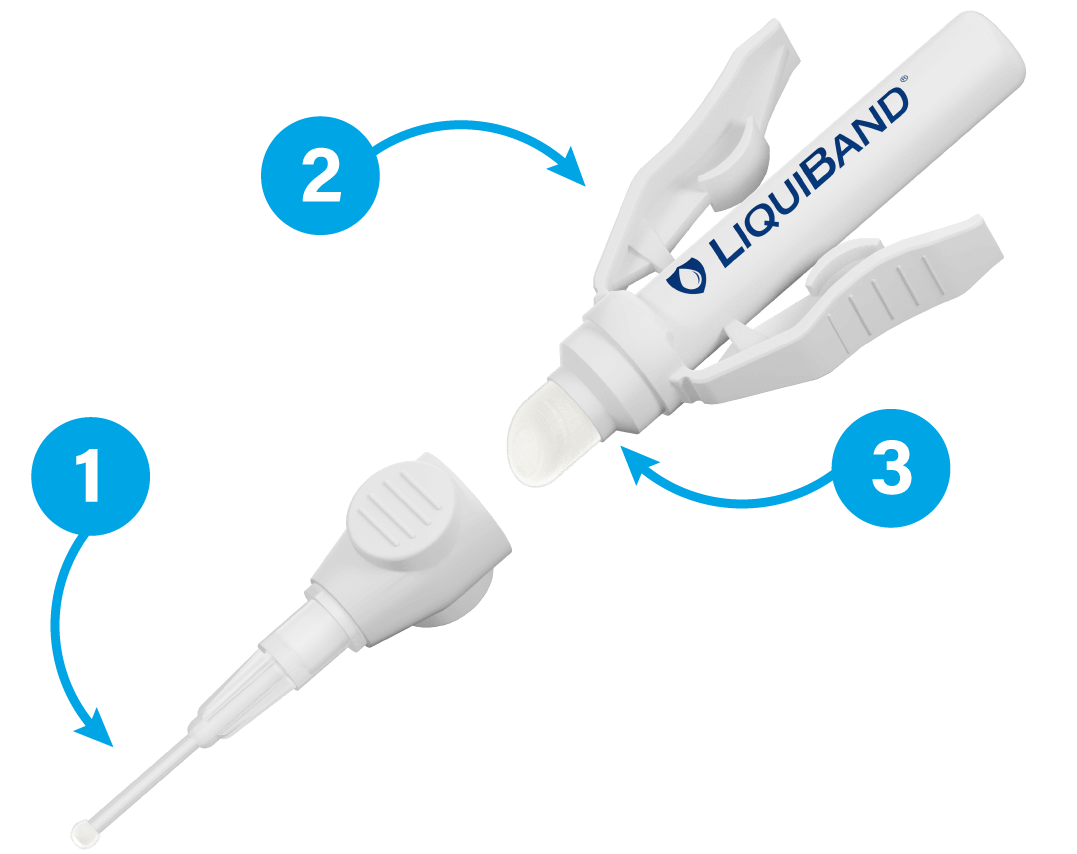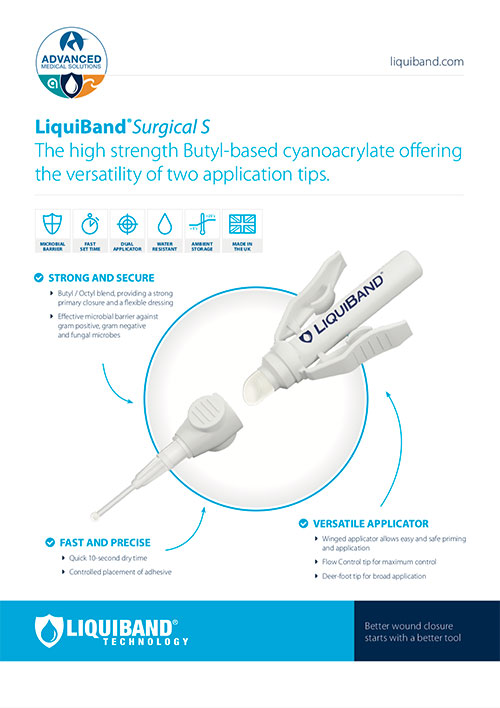
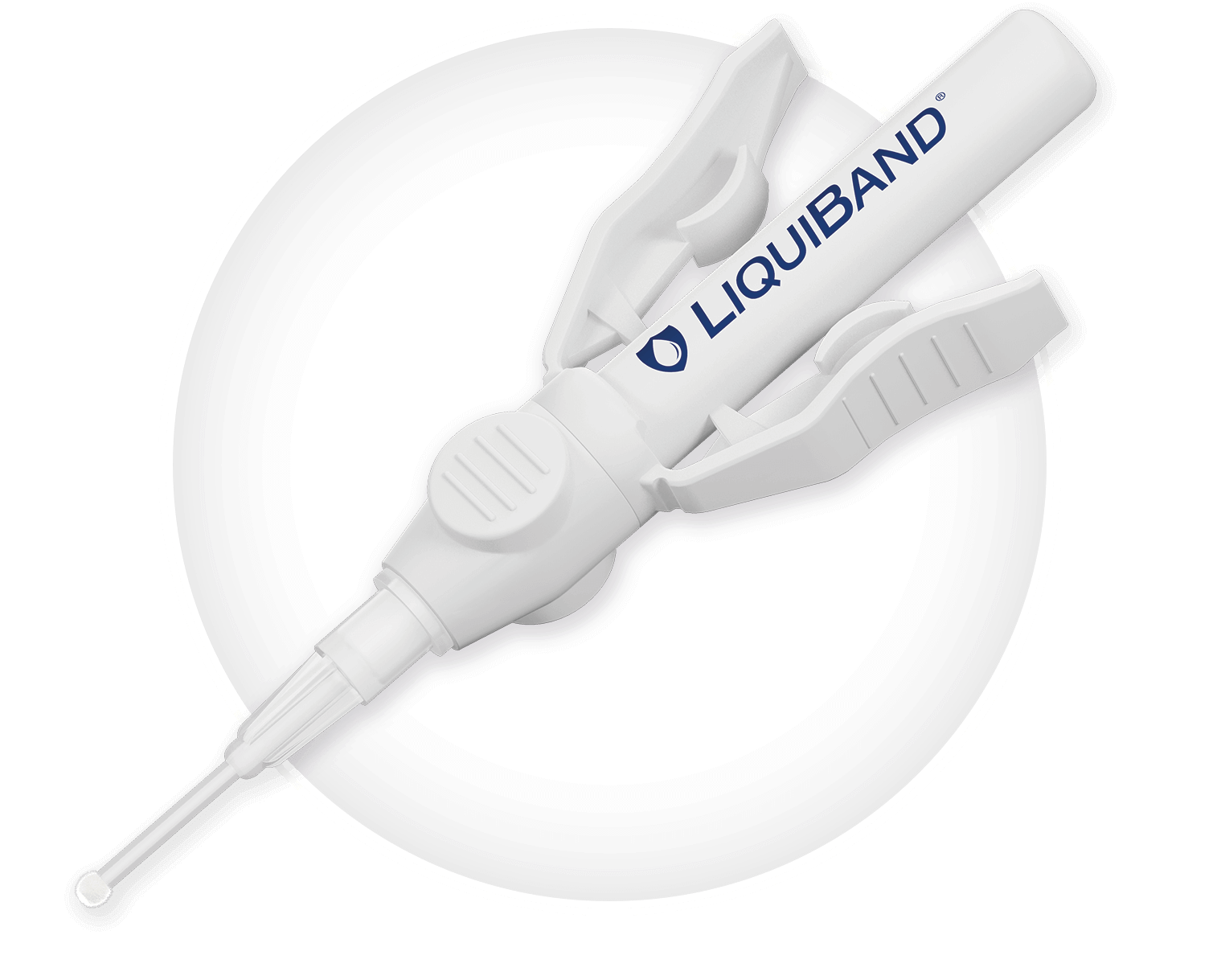
High strength Butyl/Octyl blend offering the flexibility of two application tips
LiquiBand® Surgical S

Key Features
LiquiBand® Surgical S provides microbial barrier protection.
Key Benefits
Fast precise closure1
- Quick and easy to use.
Cost effective2
- No topical sutures required, eliminates the added burden of suture removal.
Strong3 and secure
- Stronger than 4-0 suture, reducing the risk of dehiscence.
Design Features
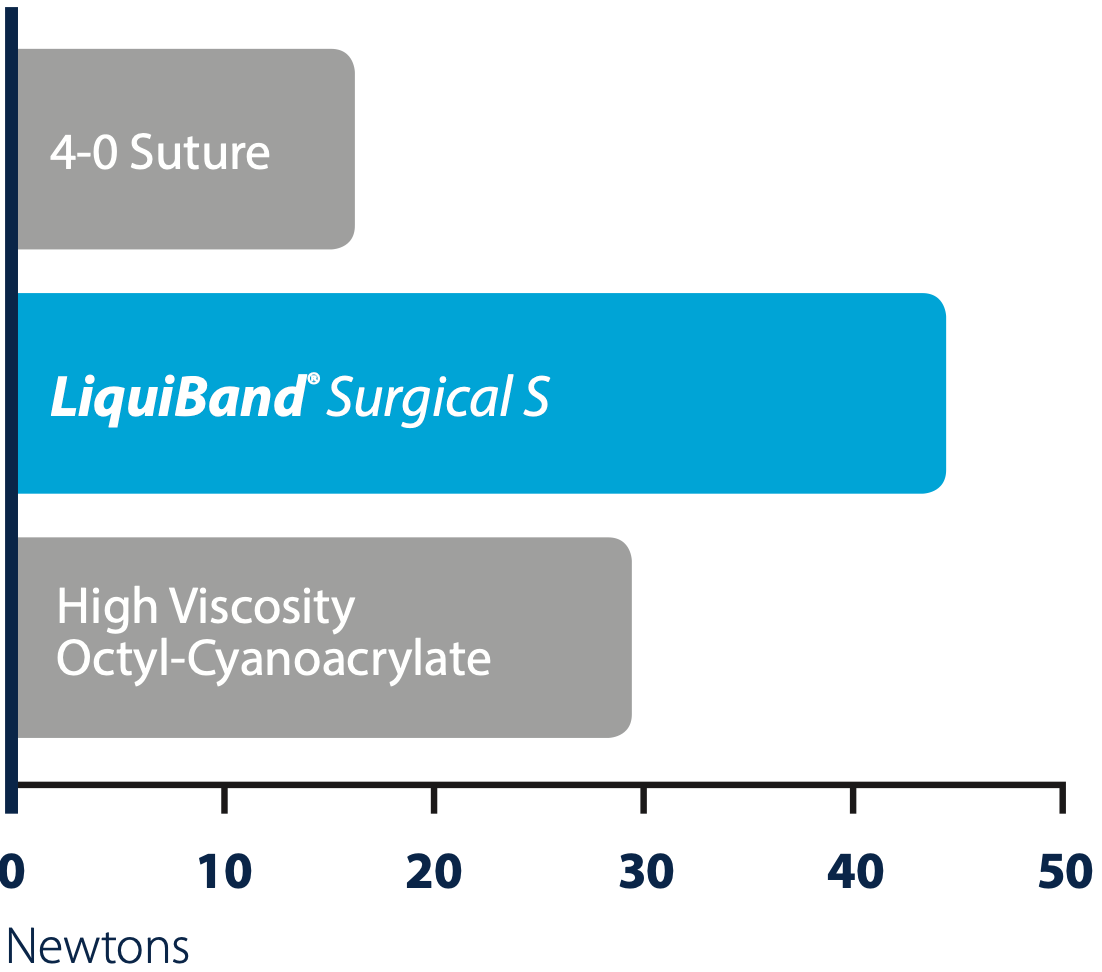
Performance
Performance
Relative strengths of topical cyanoacrylates and sutures4
Reliable wound closure
and protection
- LiquiBand® Surgical S contains a sterile blend of 90% Butyl and 10% Octyl cyanoacrylate tissue adhesive.
- Can be used to close topical incisions after a variety of surgical procedures including neuro surgery, vascular surgery, plastic surgery and orthopaedic surgery.
Sizes & Codes
LiquiBand® Surgical S
| Product | Product Code | Volume per Each | Each per Box |
|---|---|---|---|
| LiquiBand® Surgical S | LBS-S 0001 | 0.8g | 6 |
| LiquiBand® Surgical S - Clear | LBS-SC 0006 | 0.8g | 6 |