LIQUIBAND® XL
Close incisions with confidence
Designed to meet both patient and clinician needs, LiquiBand® XL is a combination product using both a mesh patch and a liquid adhesive to securely hold large and high-tension incisions closed for up to 14 days.
Water-resistant, allowing patients to shower immediately if directed by the physician7
Provides an effective barrier against gram-positive, gram-negative, and fungal microbes for as long as the film remains intact4, 8
Design features
LiquiBand® XL provides safe, secure, and effective closure for large and high-tension incisions
Simple, Ergonomic Applicator
- Signature LiquiBand® winged applicator for safe and efficient priming
- Wide tip for broad adhesive coverage
- Foam tip allows for controlled application

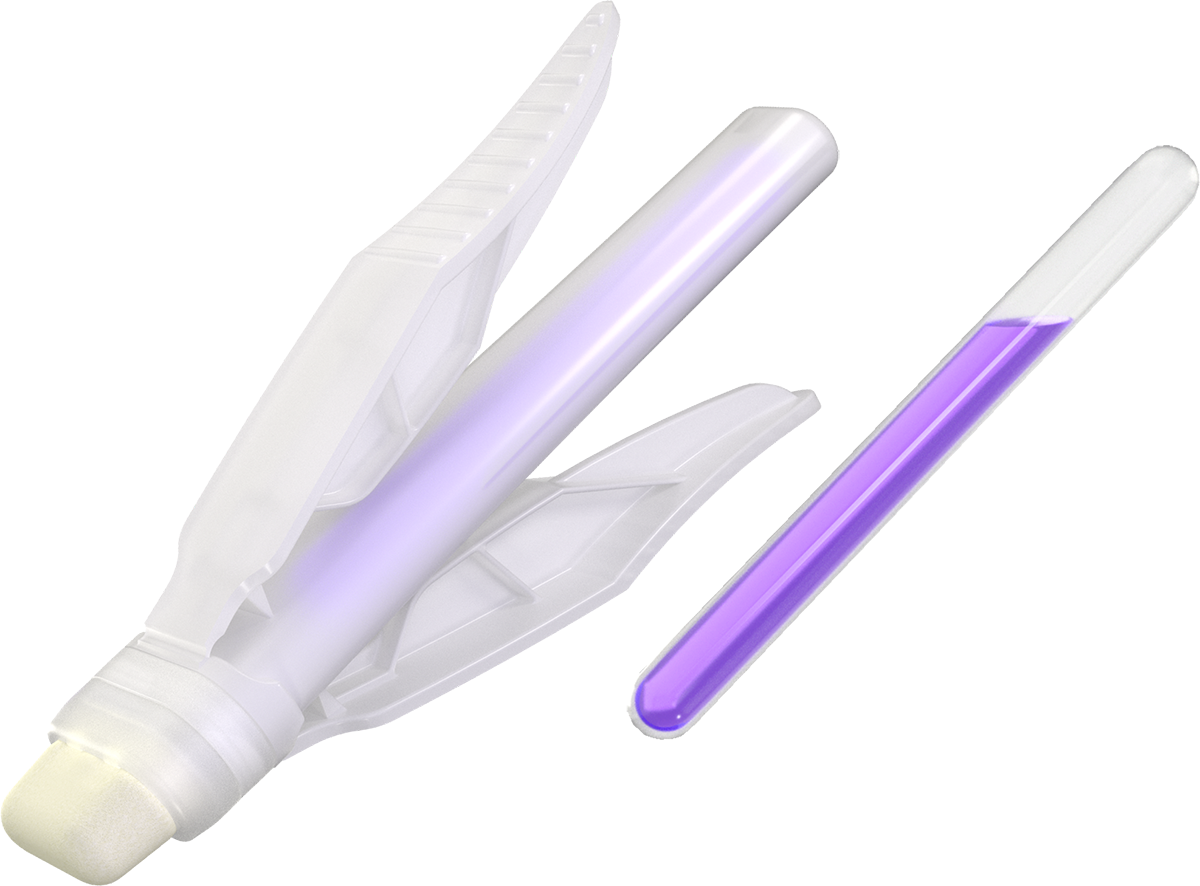
Octyl-based Cyanoacrylate Formula
- Strong and durable incision closure1-3
- Consistent dry time in as little as 60 seconds2, 3
- Proven durability for up to 14 days2, 3
- Effective microbial barrier4, 8
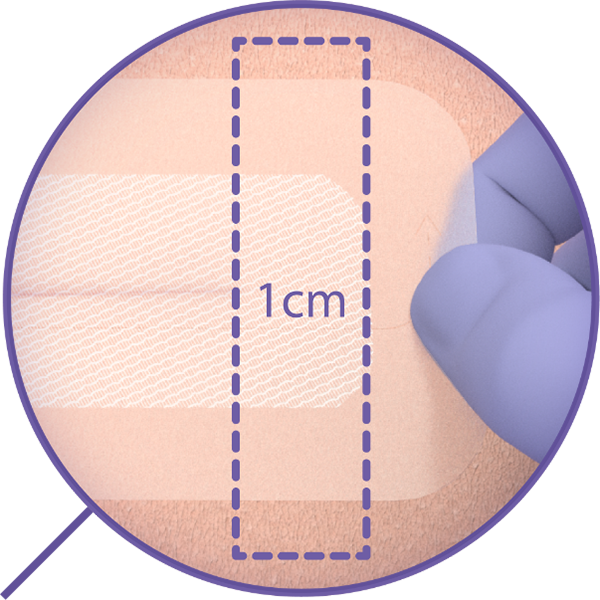
Intuitive Mesh Design
- User-friendly and easy mesh application5
- Clear mesh release liner for proper incision visualization2, 3
- Highly-visible release tab for precise release liner removal5
- Woven mesh with hexagonal pattern

Watch our video
See what LiquiBand® XL can do for you

Key benefits
Designed to meet both patient and physician needs, LiquiBand® XL offers a variety of benefits
For Patients
Provides a water-resistant microbial barrier against gram-positive, gram-negative, and fungal microbes, allowing patients to shower with physician approval.4, 7, 8
Non-invasive incision closure system that does not puncture the skin, unlike sutures or staples.2, 3
No track marks from topical sutures or staples.2, 3
Provides security for everyday activity.2, 3


For Clinicians
Provides durable closure for up to 14 days on large or high-tension incisions.2, 3
Dries consistently in as little as 60 seconds.2, 3
Secondary dressings are not required.
Procedures
LiquiBand® XL can be used on the following procedure types:
Orthopedics
Cardiothoracic
OB-GYN
Plastics
General Surgery
Spinal
Performance
25.3N
Wound Closure Strength Wound closure strength testing measures the amount of tension required to reopen a wound after device application. Higher wound closure strength results indicate a reduced risk of the wound dehiscing.
LiquiBand® XL provides equivalent wound closure strength to Dermabond® Prineo® and Exofin Fusion® 9
LiquiBand® XL: 25.36N, Dermabond® Prineo®: 22.62N, Exofin Fusion®: 21.12N
Values in (N).
46Seconds
Dry Time
LiquiBand® XL dries faster than Dermabond® Prineo® and Exofin Fusion® 9, 10
LiquiBand® XL: 46 seconds, Exofin Fusion®: 66 seconds, Dermabond® Prineo®: 175 seconds
Values in (seconds).
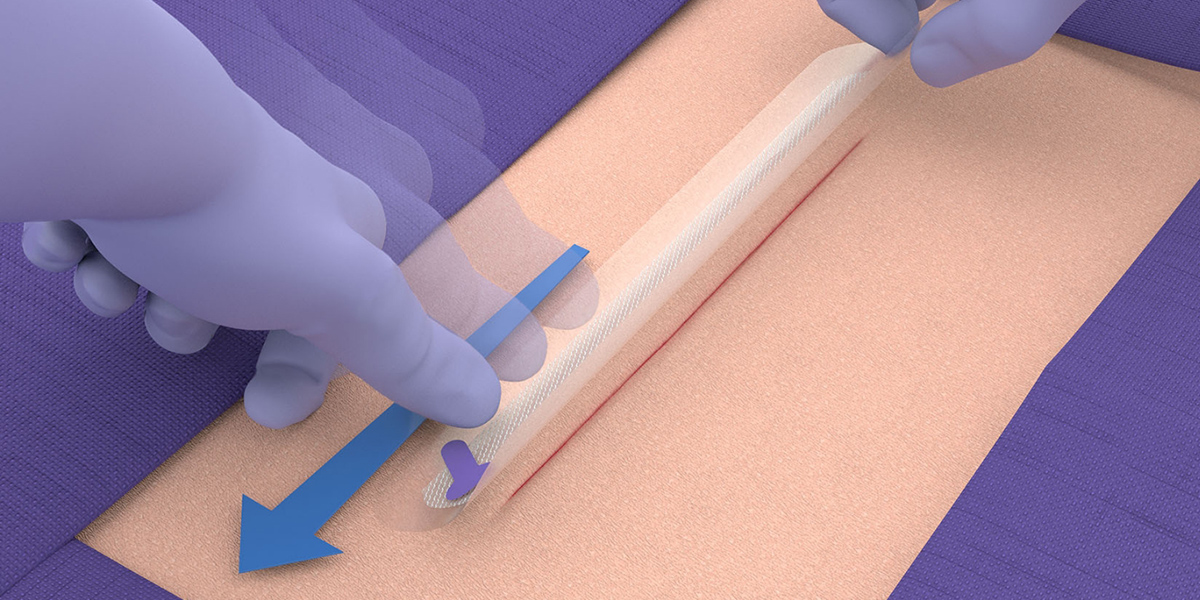
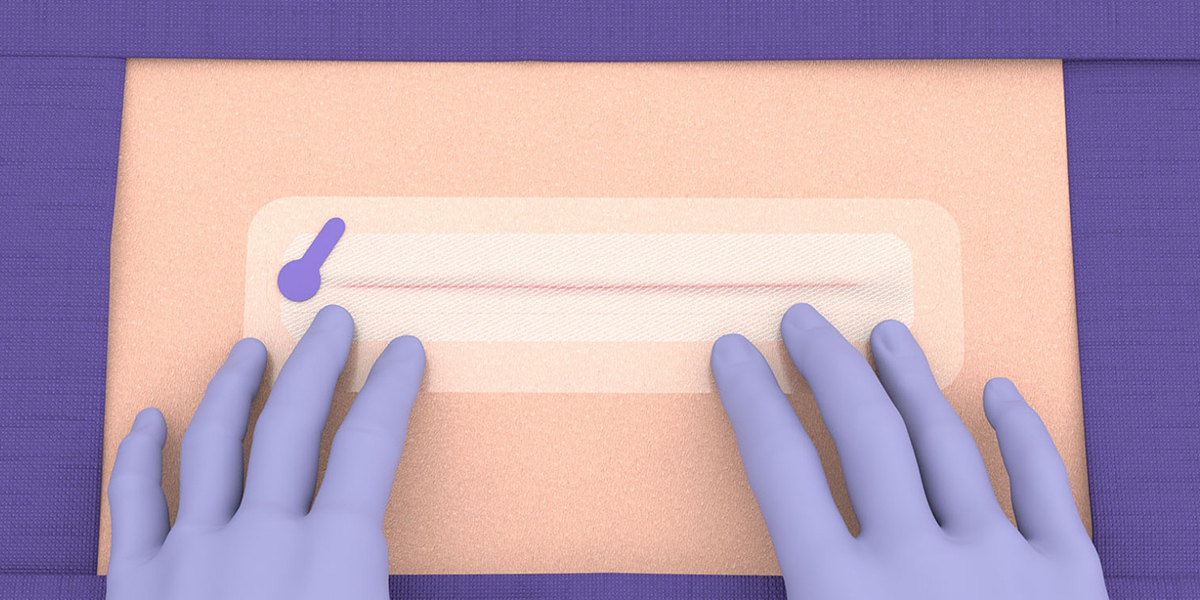
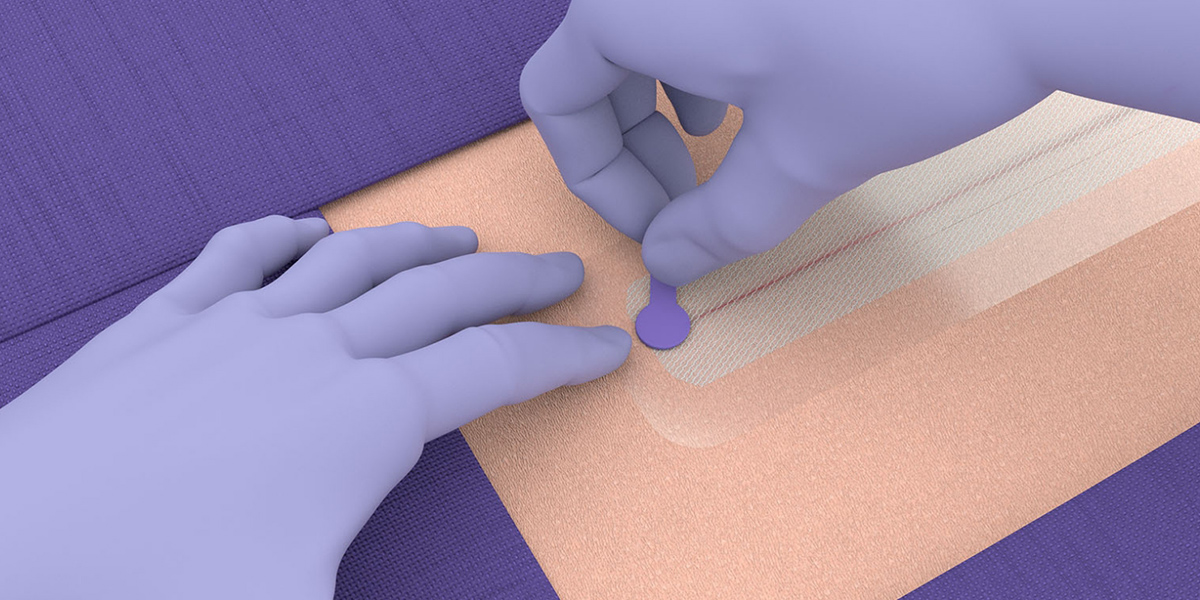
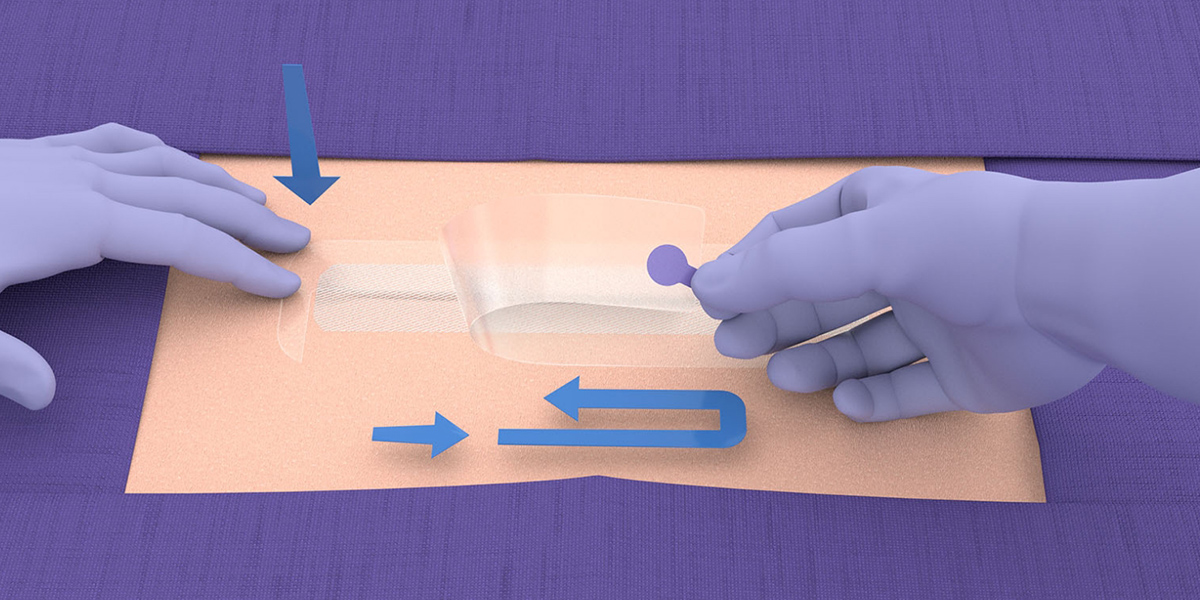
Application guide
Preparation
- Please refer to the instructions for use for comprehensive instructions and warnings for LiquiBand® XL.
- Application of the LiquiBand® XL system requires thorough cleansing of the incision.
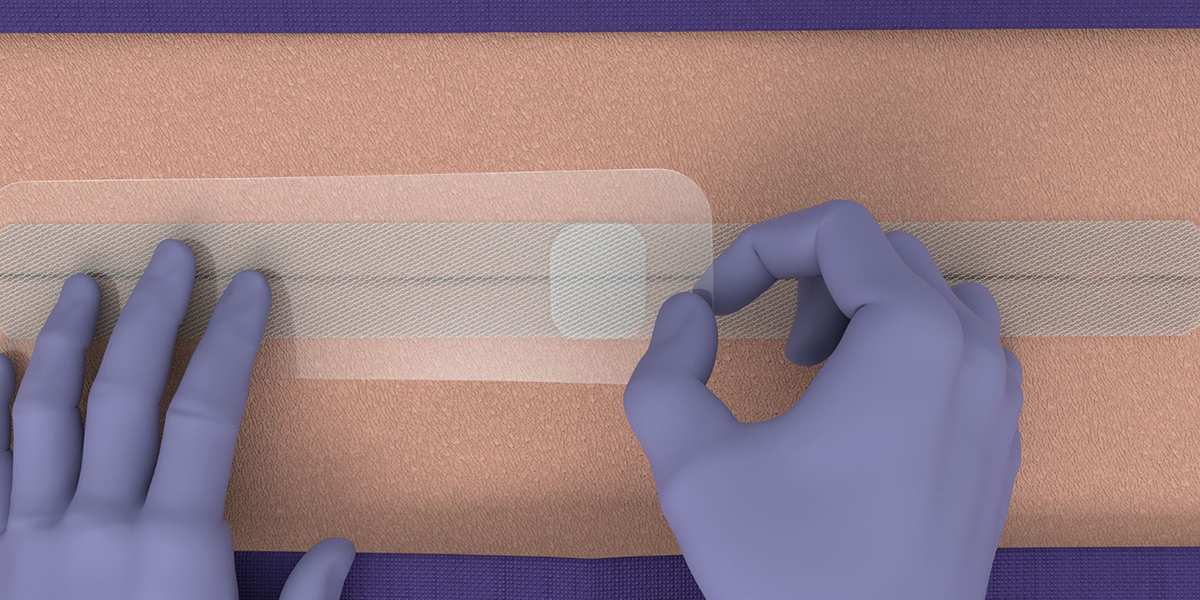
- Follow standard surgical practice for incision preparation before application of LiquiBand® XL (i.e. cleanse, irrigate, debride, obtain hemostasis, and close deep layers such that there is no tension on the skin). Skin edges must be closely approximated prior to application of the mesh, such that significant manual approximation is not required during mesh application.
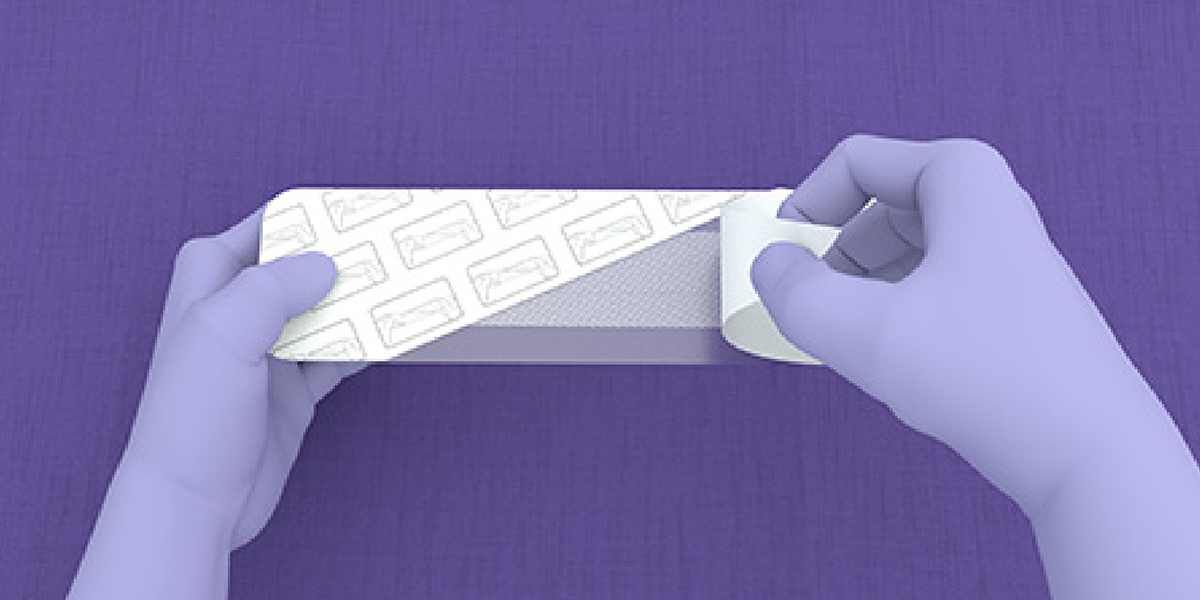
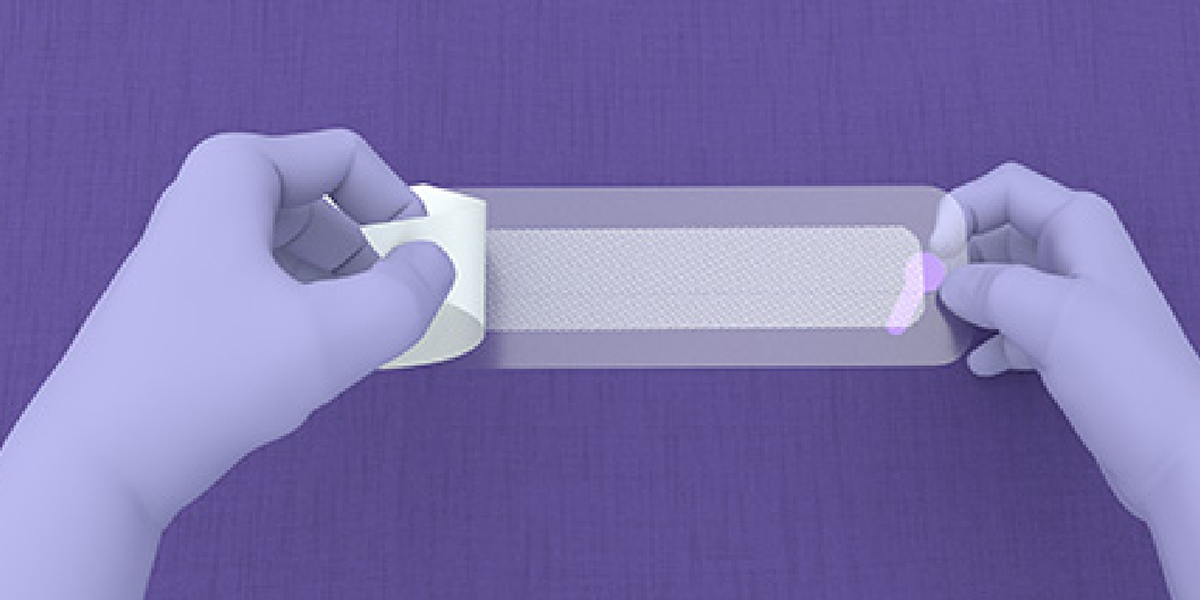
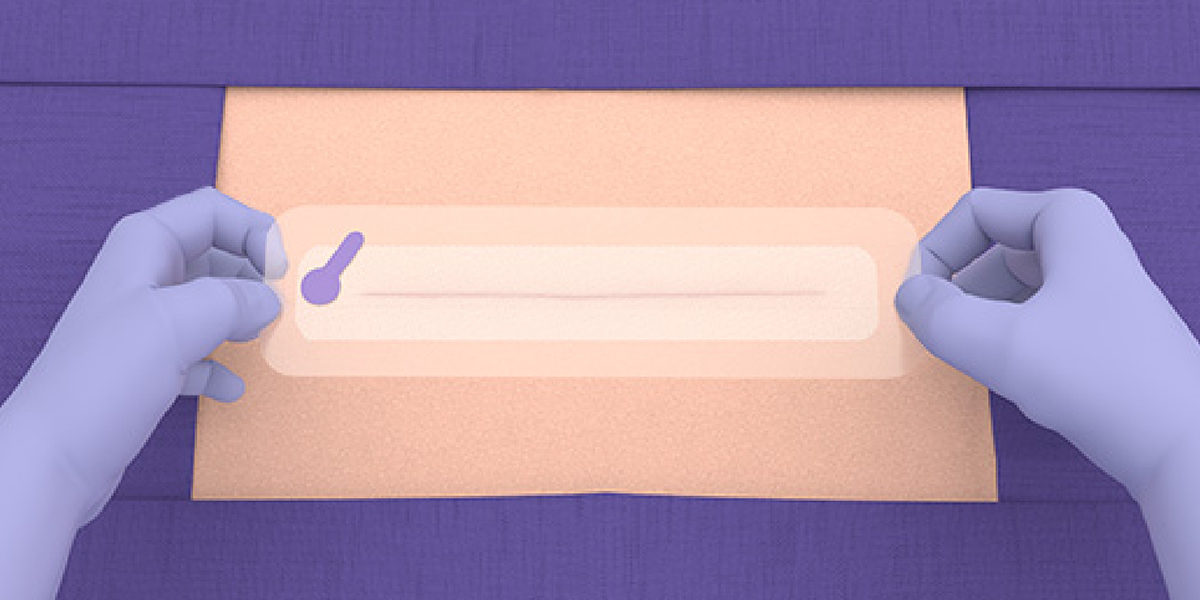
- Using sterile technique, open the packaging to access the device components.
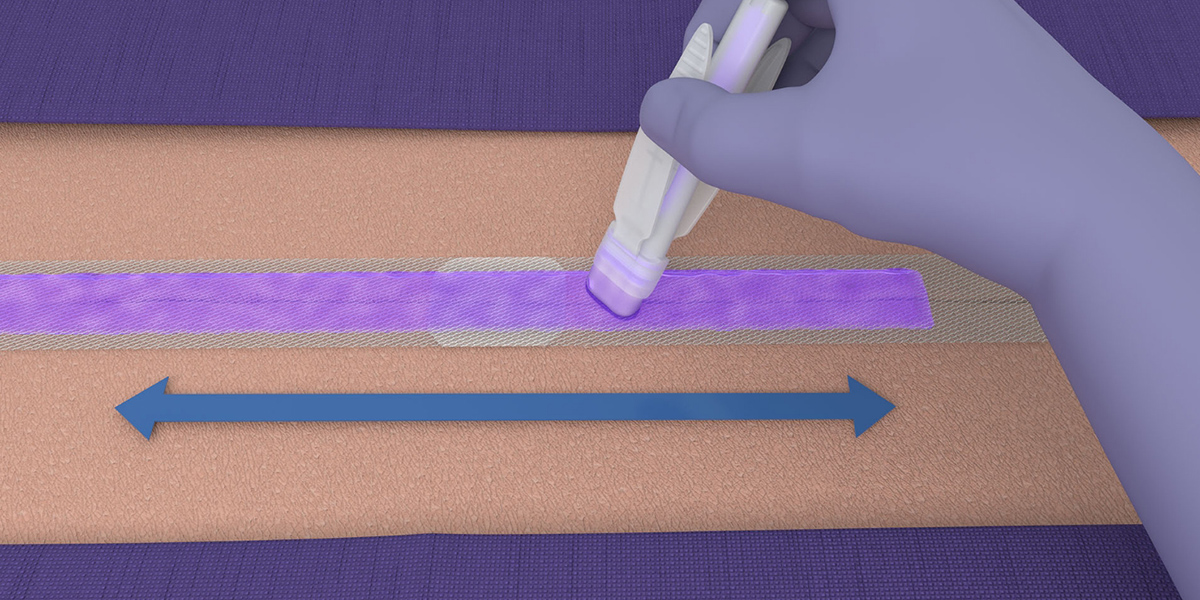
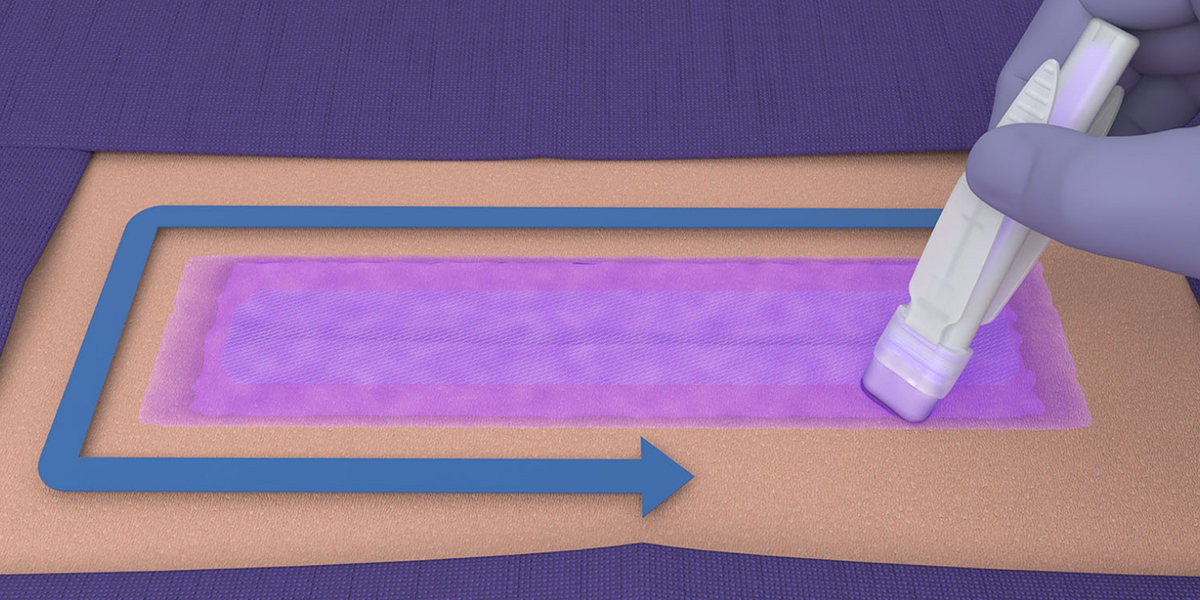
- LiquiBand® XL is applied in a 2-step process with mesh patches and applicators for dispensing the liquid adhesive.
Application

Sizes & Codes
| LiquiBand® XL | ||||
|---|---|---|---|---|
| Product Code | Qty/Box | Description | ||
| 72014019 | 2 | One 22cm x 4cm Mesh Strip, One 2.7g Adhesive Applicator | ||
| 72014027 | 2 | Two 22cm x 4cm Mesh Strips, Two 2.7g Adhesive Applicators | ||
| 72014046 | 2 | Three 22cm x 4cm Mesh Strips, Three 2.7g Adhesive Applicators | ||
Downloads

LiquiBand® XL Brochure UK
(PDF - 2MB)

LiquiBand® XL Application Guide English
(PDF - 2MB)
LiquiBand® Skin Reactions Brochure
(PDF - 3.6MB)
LiquiBand® XL Clinical Pathway Guide English
(PDF - 1MB)
Contact us for more information
References
1 - 10: All data on file at Advanced Medical Solutions Limited